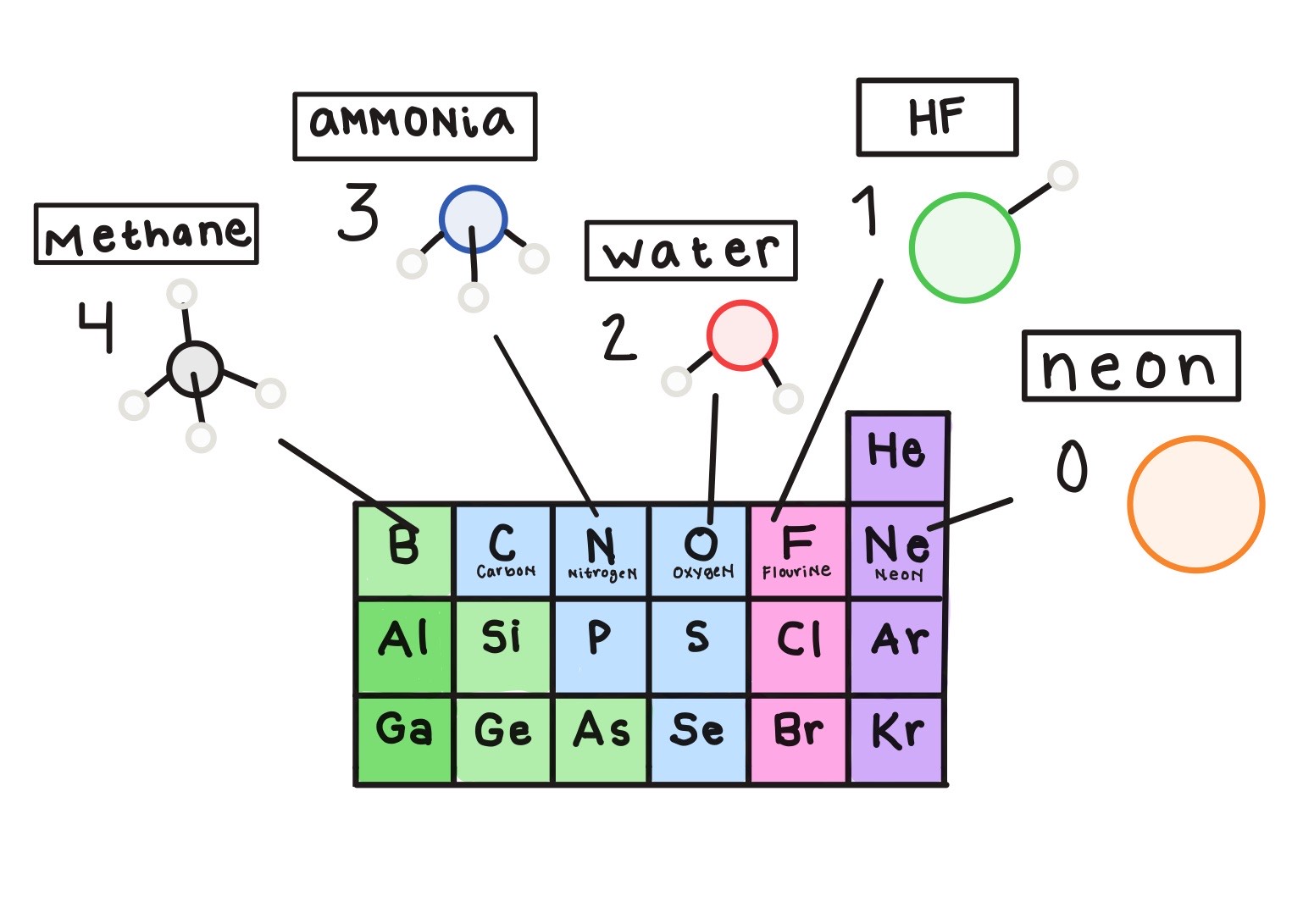
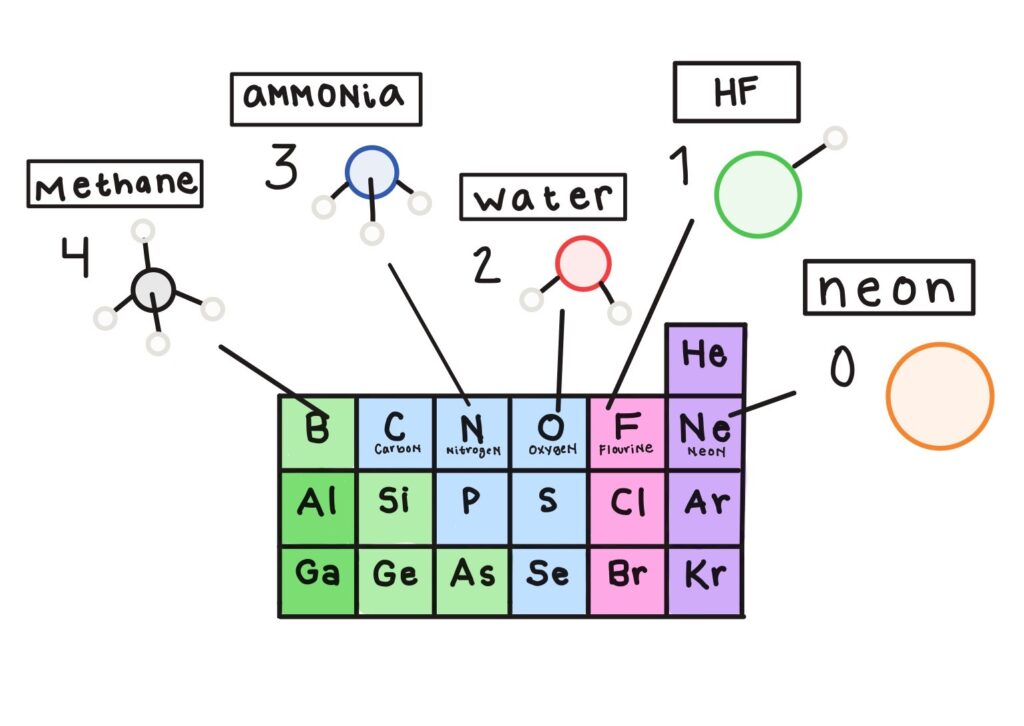
Hello! My name is Serena and I’m currently a master’s student in theoretical and computational chemistry as a part of the Erasmus Mundus Joint Master’s program in Europe. A few weekends ago, some classmates and I decided to venture out of our comfort zone and participate in IBM’s Qiskit Fall Fest 2025 Hackathon. The purpose was to use Qiskit, a quantum software development kit from IBM, to solve a problem using quantum computation instead of classical computers over the course of four days.

Day 1 – Introduction & Talks
First task—meeting everybody. The first day, we gathered in the physics faculty at the University of Barcelona to introduce ourselves to each other and to the new quantum concepts we would be using. Artur Garcia gave the first talk from the Barcelona Supercomputing Center; he discussed tensor networks for circuit simulation and a little bit about the difference between classical supercomputing and quantum computing. He was followed by Niccolo Baldelli, also from the Barcelona Supercomputing Center, who gave a great presentation on simulating quantum circuits.
Day 2 – Set Up
The next day, we were on our own; we had to install the Qiskit package on our computers and complete an exercise to familiarize ourselves with the software. They sent the instructions over Discord, which included a link to IBM’s YouTube channel that walked you through the entire setup procedure and one practice problem. By the end of the night, I was ready and nervous; I was about to spend 48 hours doing something I had never done before.
Day 3 – Hacking!
Joana Fraxanet from IBM came in the morning to kick us off with a presentation on quantum algorithms and their applications (shoutout quantum chemistry!). She gave us more information about the connection between high performance computing and quantum computing, and advised us on the quantum algorithms we would use. Then it was time for the challenges!

My group chose the intermediate challenge of the three options called “The Queen’s Problem”. Although none of us had ever used Qiskit before, we thought trying a challenge that interested us was the way to go: the best motivator is genuine curiosity! At about 11:00, we set out for a full day of coding (eight hours!), stopping only at 2:00 pm for a quick lunch break.
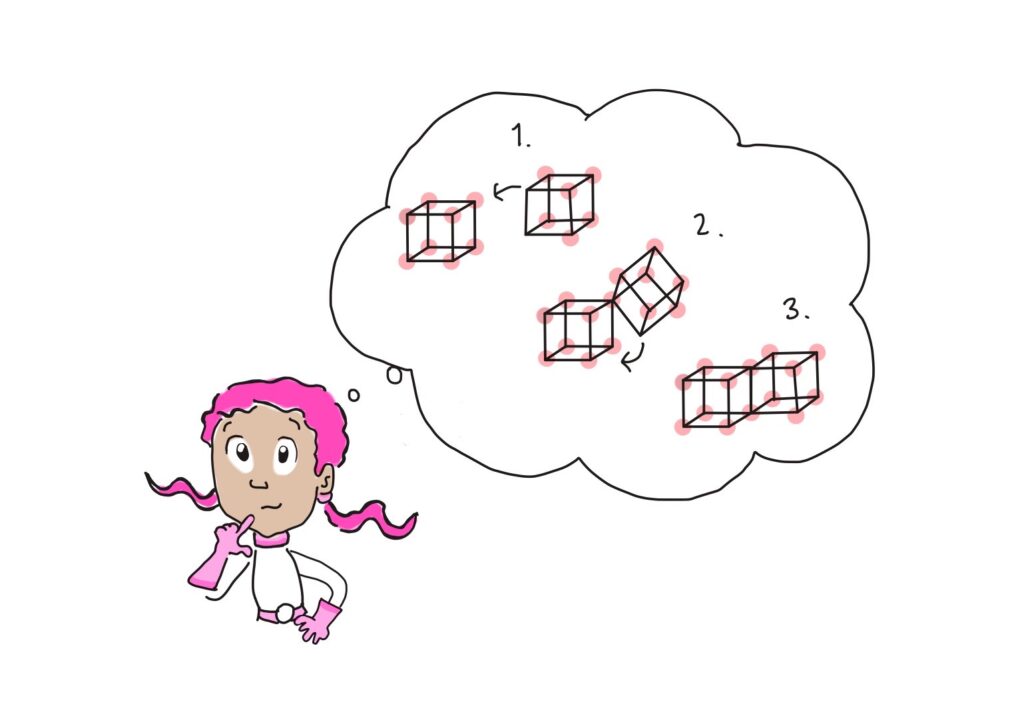
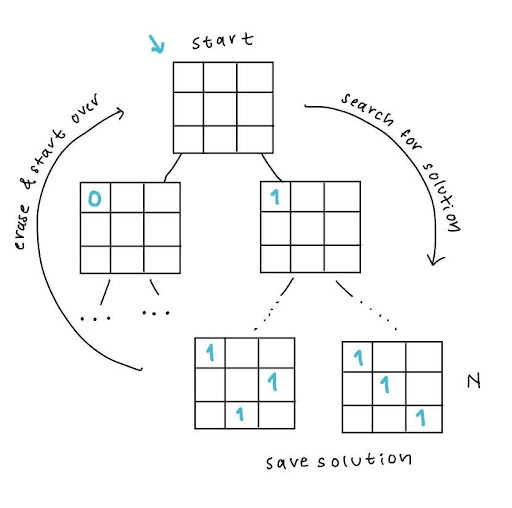
The challenge we had selected was this: find the maximum number of queens you can place on a chessboard so that no two queens can attack each other. For the first part of the challenge, we had to use a classical algorithm, or a brute-force method, to find the answer to this question. We started with a blank chessboard, filled in the first eligible space with a piece, and then had the code find all the possible solutions from there. After it saved all the solutions from that starting point, it saved them, wiped the board, and started again.
The next part of the challenge used a quantum algorithm to transform the problem into a physics one: we were going to use a “lowest energy state” to find a board with no pieces attacking each other. The lowest energy state in physics just means the point in the system with the lowest energy. If our system is a ball teetering at the top of a valley, the highest energy state is going to be the ball at the top, while the lowest energy state is going to be the ball at the bottom. If you give that teetering ball a push, where is it going to go? That’s our lowest energy state.
The quantum algorithm would basically do the same thing with the arrangement of the board; if it placed pieces that could attack each other, that would be a high-energy state, and if they couldn’t, it would be a low-energy state. The goal was to have the algorithm place pieces to find, like our ball rolling down the hill, in the lowest possible energy state.
The hardest part about this challenge for me was translating my logic into code. I could answer the question on paper, and on paper it seemed so simple, but then I had to execute it using Qiskit–something I had never done before. It was a lot of trial and error (and a lot of asking for help), but by the end of the day, we were getting the hang of it.

Day 4 – Presentations & Results
The next day, I was back to hacking after coffee and breakfast. We took our seats and dove back into where we left off. The day before, we had completed the challenge for the rook and bishop pieces. Today was about putting it all together to solve the queen’s question.
By lunch, we were almost finished, but had to shift our attention to designing our presentation. In terms of the competition, how we presented our solutions was almost as important as what those solutions were.
Finally, at five, all eight teams were ready to present. We watched as groups presented their solutions for the various challenges, and presented as they listened to ours. Our group successfully completed the part of the challenge that used classical computers to solve the queens’ problem, but only got about two-thirds of the way through the quantum one. We wrote the quantum algorithm we needed to solve it, it just wasn’t giving us the right answers! Still, not bad for a group of chemists among physicists.
In the end, the prize went to a very deserving team that worked on the hardest challenge, the Phase Recognition Challenge, which had to do with identifying the phase of a quantum state, or how a quantum state evolves over time.
My biggest takeaway from the event was: ‘I should have done this sooner’. In college, I always prioritized the acquisition of knowledge over its use. Even though I’m technically learning programming as a part of my curriculum right now, I learned so much in just 48 hours by being forced to use and apply it. Doing it with my friends and working on a real problem I found interesting helped too.

So, if you are interested in learning quantum mechanics, my advice is to get involved: go to an event or try out some online resources. Even better to do it with a friend.
If you’re interested in learning more about quantum computing or checking out Qiskit for yourself, here are some of the resources we used over the weekend:
- IBM Quantum Computing – Qiskit
- Quickstart | Getting Started with Qiskit
- Coding with Qiksit | Youtube Tutorial
Serena Krejci-Papa is a first-year master’s student at the University of Barcelona, studying theoretical and computational chemistry with the Erasmus Mundus program. She writes about complex science topics in a way that makes people laugh. You can find more about her at Sciencewithserena.com.