What Quantum Mechanics Taught Us About Feeding Half the World
(Olivia Castillo is a physics student and APS JNIPER fellow.)
During the first decade of the 20th century, a German chemist named Fritz Haber pulled bread out of thin air, feeding the hungry mouths of a rapidly growing population and ultimately saving billions from starvation. He discovered a method to transform our nitrogen-rich air into ammonia, which fertilizes so much agriculture that half of the world’s population is dependent on it.
Although synthesizing ammonia from air may seem straightforward due to the presence of only two essential ingredients (nitrogen and hydrogen), it is a process that is remarkably complex. This is largely due to the fact that molecular nitrogen has a triple bond, one of the strongest in chemistry, and breaking it requires a tremendous amount of energy.
It’s worth noting that ammonia is naturally produced by lightning, a phenomenon that is notoriously difficult to replicate in a controlled lab setting, underscoring the enormity of the challenge. To artificially manufacture ammonia, Haber incorporated a catalyst, a substance that offers an alternative pathway with lower energy requirements to speed up reactions. With the catalyst, Haber satisfied a ravenous population, but Haber’s breakthrough had a blind spot: how it actually worked.
In the absence of this knowledge, Haber’s successors turned to a familiar scientific strategy, relentless trial and error. One notable example was Alwin Mittasch, a German chemist, who conducted roughly 20,000 experiments between 1909 and 1912 to test possible catalysts. Although these educated guesses greatly enhanced Haber’s method, it was still a mystery how each of those catalysts affected the reaction efficiency.
For early ammonia production, this iterative experimentation worked wonderfully to roll out the ammonia factories and nourish the population. But now, about 2% of the entire globe’s energy consumption is dedicated to ammonia synthesis. As we flood the atmosphere with greenhouse gases, we need a sharper understanding to reduce the reaction’s energy consumption and maximize its efficiency. Thankfully, quantum mechanics fulfilled this need.
Mapping the Catalyst’s Surface: A Treasure Hunt for Chemists
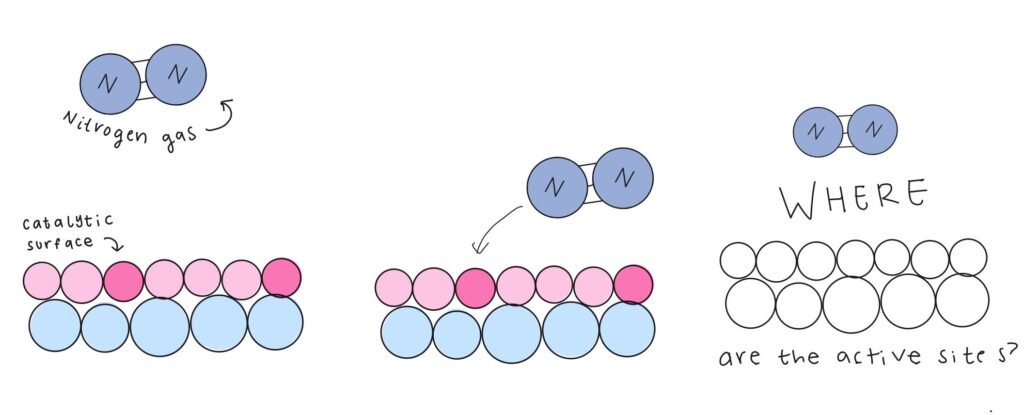
Common types of catalysts are pieces of metal or alloys that are not consumed by the reaction, remaining unchanged, and it is on their surface where the desired reaction occurs. The main culprit behind early chemists’ lack of understanding of the ammonia synthesis was the intricate surface of the catalyst. These surfaces need to be mapped out diligently because, like a pirate searching for a treasure chest, scientists hunt for active sites, precise points where the chemical reaction takes place, scattered along the surface. An accurate map of the landscape reveals these treasures, allowing chemists to pinpoint where the reaction occurs and illuminating how the catalyst supports the chemical transformation.
In the case of ammonia synthesis, for nitrogen gas to react, it must anchor onto active sites, breaking its own internal triple bond and replacing it with a bond to the catalyst. To increase the chances of this happening, small particles of the catalyst are sprinkled into a highly porous supporting material, providing a larger surface area for the nitrogen gas to bind to.

However, because nitrogen can only stick to rare spots on the catalyst, it is difficult for scientists to locate these active sites upon mostly barren terrain. Furthermore, other atoms bound to the catalyst’s surface could affect its reactivity and complicate the surface characterization. To answer these questions, a new kind of map would need to be developed, one drawn by quantum mechanics.
Using Quantum Mechanics to Model What We Can’t See
In 2005, Karoliina Honkala and her colleagues from the Center for Atomic-Scale Materials Physics in Denmark, equipped with a powerful computational tool from quantum mechanics—Density Functional Theory (DFT)—investigated the surface of ruthenium. Ruthenium is the most effective catalyst for manufacturing ammonia, although iron is the most widespread due to its lower cost.
Quantum mechanics is the branch of physics that describes atomic interactions, and its foundation lies in Schrödinger’s equation, which chronicles how the quantum state of a system evolves over time. Like the sophisticated algorithm running behind your TikTok feed, Schrödinger’s equation runs the show in quantum physics. But the Schrödinger equation is incredibly difficult to solve. Thus, DFT solves an approximate version of the Schrödinger equation. By finding solutions to this potent equation, DFT allows scientists to zoom into the tiny world of atoms without having to direct X-rays at them or perform any other physical experiments.
In the study by Honkala and her team, DFT first confirmed that breaking nitrogen’s triple bond was the most sluggish and energy-demanding step of ammonia synthesis. More importantly, Honkala and her colleagues estimated the number of active sites on the catalyst as a function of its size, a novel breakthrough in the field. As a sanity check for their model, they determined the overall rates of ammonia production under industrial conditions, which agreed incredibly with known values. This level of detailed insight is valuable for catalyst design and optimization, and over half a dozen patents have sprung out of this study.
What made their findings revolutionary was that they did not rely on any experimental values to fit their model, only using quantum mechanics through DFT to understand the reaction.
The multiple uses of the quantum tool DFT
From uncovering the structure of Earth’s iron core (too deep for any measuring probe to reach) to ruling out trial materials unlikely to become effective drugs, DFT has been advancing science in the background for decades, elucidating complex systems with impressive accuracy.
Many people believe quantum mechanics yields random, uninterpretable results. But despite its statistical nature and philosophical puzzles, quantum mechanics has delivered tangible, impactful results that shape our world for the better. DFT is proof of that: without synthetic ammonia fertilizers—enabled by catalysts like the one Honkala and her team studied—nearly 4 billion people would go hungry.
References
Main study of this article
Honkala, K., et al. “Ammonia Synthesis from First-Principles Calculations.” Science (American Association for the Advancement of Science), vol. 307, no. 5709, 2005, pp. 555–58, https://doi.org/10.1126/science.1106435.
A follow-up study with Honkala as a coauthor that provided a lot of useful information for this article
Hellman, A., et al. “Predicting Catalysis: Understanding Ammonia Synthesis from First-Principles Calculations.” The Journal of Physical Chemistry, vol. 110, no. 36, 2006, pp. 17719–35, https://doi.org/10.1021/jp056982h.
Book chapter on DFT that inspired this article, containing useful explanation of the main study
Sholl, David S., and Janice A. Steckel. “What is Density Functional Theory?” Density Functional Theory : A Practical Introduction / David S. Sholl and Jan Steckel. 1st ed., Wiley, 2009.
Article about DFT and Earth’s Inner Core
Cote, A. S., et al. “Ab Initio Lattice Dynamics Calculations on the Combined Effect of Temperature and Silicon on the Stability of Different Iron Phases in the Earth’s Inner Core.” Physics of the Earth and Planetary Interiors, vol. 178, no. 1–2, 2010, pp. 2–7, https://doi.org/10.1016/j.pepi.2009.07.004.
Statistics about Ammonia’s Energy Consumption
IEA. “Ammonia Technology Roadmap.” The International Energy Agency, 2021, https://www.iea.org/reports/ammonia-technology-roadmap. Accessed 27 June 2025.
Data about Ammonia Fertilizer Use
“World Population With and Without Synthetic Nitrogen Fertilizers.” Our World in Data, 2015, https://ourworldindata.org/grapher/world-population-with-and-without-fertilizer. Accessed 27 June 2025.
Photo of Karoliina Honkala: Photo taken by Petteri Kivimäki. Provided by Karoliina Honkala.
Written by Olivia Castillo, a senior, studying physics and humanities at the University of Texas at Austin.
Illustrations created by Serena Krejci-Papa, a first-year master’s student at the University of Barcelona, studying theoretical and computational chemistry with the Erasmus Mundus program. She writes about complex science topics in a way that makes people laugh. You can find more about her at Sciencewithserena.com.
For general questions about IYQ, please contact info@quantum2025.org. For press inquiries, contact iyq2025@hkamarcom.com.




