The History of Light and the Birth of Quantum Physics
(Richard Sottie is a physics student and APS JNIPER fellow.)
The quest to understand the fundamental nature of light has sparked debate for centuries and ultimately played a pivotal role in the development of quantum physics. Even the earliest recorded speculations about light contained the seeds of concepts that would, centuries later, be woven into our quantum understanding of reality.
Ancient and Medieval Foundations of Optics (6th Century BCE – 11th Century CE)
Some of the earliest recorded ideas about the nature of light appeared in the 6th century BCE. In India, for instance, the Vaisheshika school of philosophy described light as consisting of fire-like particles moving at a high speed.
In ancient Greece, the Pythagoreans (6th–5th century BCE) and later Euclid (c. 300 BCE) advocated the emission theory of vision, suggesting that rays of light emanate from the eyes toward objects. In contrast, Epicurus (341–270 BCE) proposed an intromission theory, arguing that light consists of material images or “eidola” emitted by objects that travel to the eyes.
Further insights into the nature of light emerged in Egypt, where Ptolemy (c. AD 90–168), working in Alexandria, conducted experiments showing that light reflects off smooth surfaces and bends when passing through transparent materials of different optical densities.
Building on these concepts, Arab scholars made significant contributions to the understanding of light by establishing foundational principles governing its behavior in lenses, mirrors, and prisms. The most influential among them was the 11th-century scholar Ibn al-Haytham, who lived in present-day Iraq. Ibn al-Haytham corrected the emission theory, stating that vision results from light entering the eye rather than emanating from it.
European Wave vs. Particle Debate (17th Century CE)
By the 17th century, European scientists were divided between two competing theories about the fundamental nature of light: whether it behaved as a wave or as a particle. Dutch physicist Christiaan Huygens argued that light propagates as waves according to his wave-front principle, which explains how waves evolve when they encounter obstacles. In contrast, Isaac Newton proposed a corpuscular theory, suggesting that light consists of particles traveling in straight paths, capable of reflecting off surfaces like mirrors. Newton’s prism experiments, which showed white light splitting into colors with distinct refraction angles, supported his corpuscular theory by suggesting that light consists of particles of different sizes, each corresponding to a different color, which bend by different amounts.
Both theories, however, were incomplete. Huygens’ wave theory struggled to fully explain why light travels in a straight path, and Newton’s particle theory couldn’t account for diffraction, the spreading of light as it passes around edges or through narrow openings. Nevertheless, Newton’s theory gained significant influence not only because of his clever prism experiments, but also because he was highly revered in the scientific community, making his ideas difficult to challenge for years.
Wave theory prevails (1801 – 1888)
But science doesn’t bow to reputation. As Richard Feynman famously pointed out in his 1964 Messenger Lectures at Cornell University, “It doesn’t matter how beautiful your theory is, it doesn’t matter how smart you are. If it doesn’t agree with the experiment, it’s wrong.” Indeed, nearly a century after Newton’s corpuscular theory of 1704, the scientific consensus gradually shifted toward the wave theory of light through decades of experiments, modeling, and debates. One of the key contributors to this shift was Thomas Young, an English polymath. In 1801, Young passed sunlight through a narrow slit to produce a coherent light beam. He then placed a thin obstacle, such as a hair or a narrow strip, across the beam, splitting it into two waves that spread out and overlapped. When these waves recombined on a screen, they produced a pattern of bright and dark bands (see Figure 1.1). This interference pattern provided clear and compelling evidence that light behaves as a wave rather than as a particle.
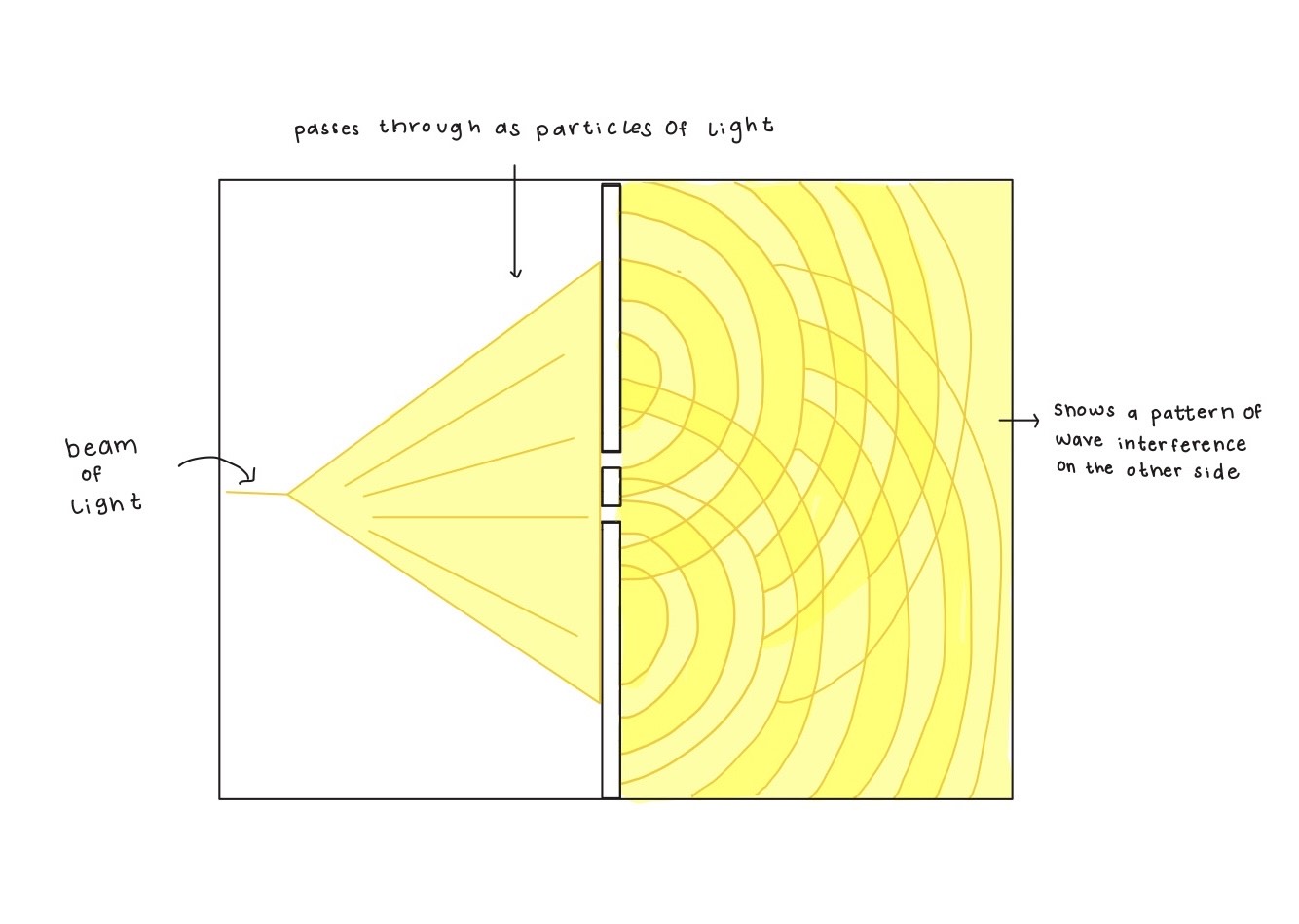
A familiar example of this phenomenon is when waves on the surface of water either combine to form larger peaks or cancel each other out. Later, in 1845, another English physicist Michael Faraday showed that magnetic fields could alter the polarization plane of light, revealing a connection between light and electromagnetism. In the 1860s, Scottish physicist James Clerk Maxwell built on this connection by formulating the theory of electromagnetism, describing light as an electromagnetic wave composed of oscillating electric and magnetic fields at right angles to each other and to the wave’s direction of travel. Heinrich Hertz’s experimental confirmation of Maxwell’s predictions in the 1880s provided further validation of the electromagnetic wave theory of light.
Resurgence of particle theory (1900 – 1923)
By the 1890s, Maxwell’s mathematical description of electromagnetic waves was considered so successful, particularly for its predictive power, that many physicists believed the fundamental nature of light was fully understood. But in the early 20th century, experimental discoveries began to challenge this consensus. The electromagnetic wave theory failed to predict how matter emits and absorbs radiation at thermal equilibrium. To address this question, German physicist Max Planck introduced a revolutionary idea known as energy quantization. He proposed that electromagnetic radiation is emitted or absorbed in discrete amounts, now called quanta, with higher frequency light corresponding to larger quanta. His theory accurately matched experimental results and earned him the Nobel Prize in Physics in 1918.
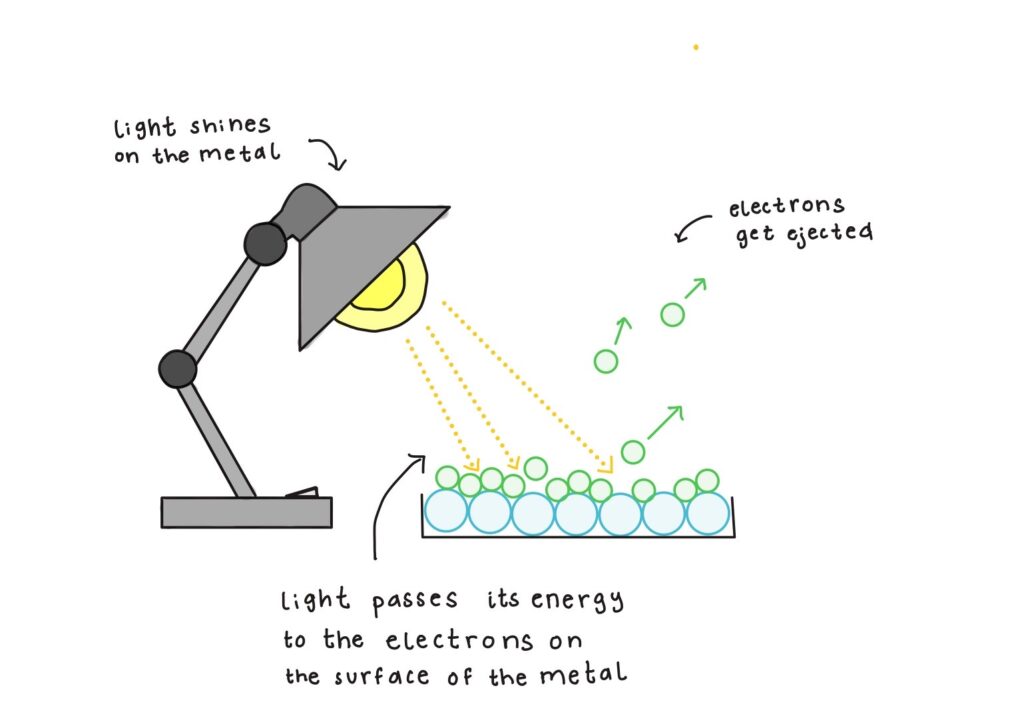
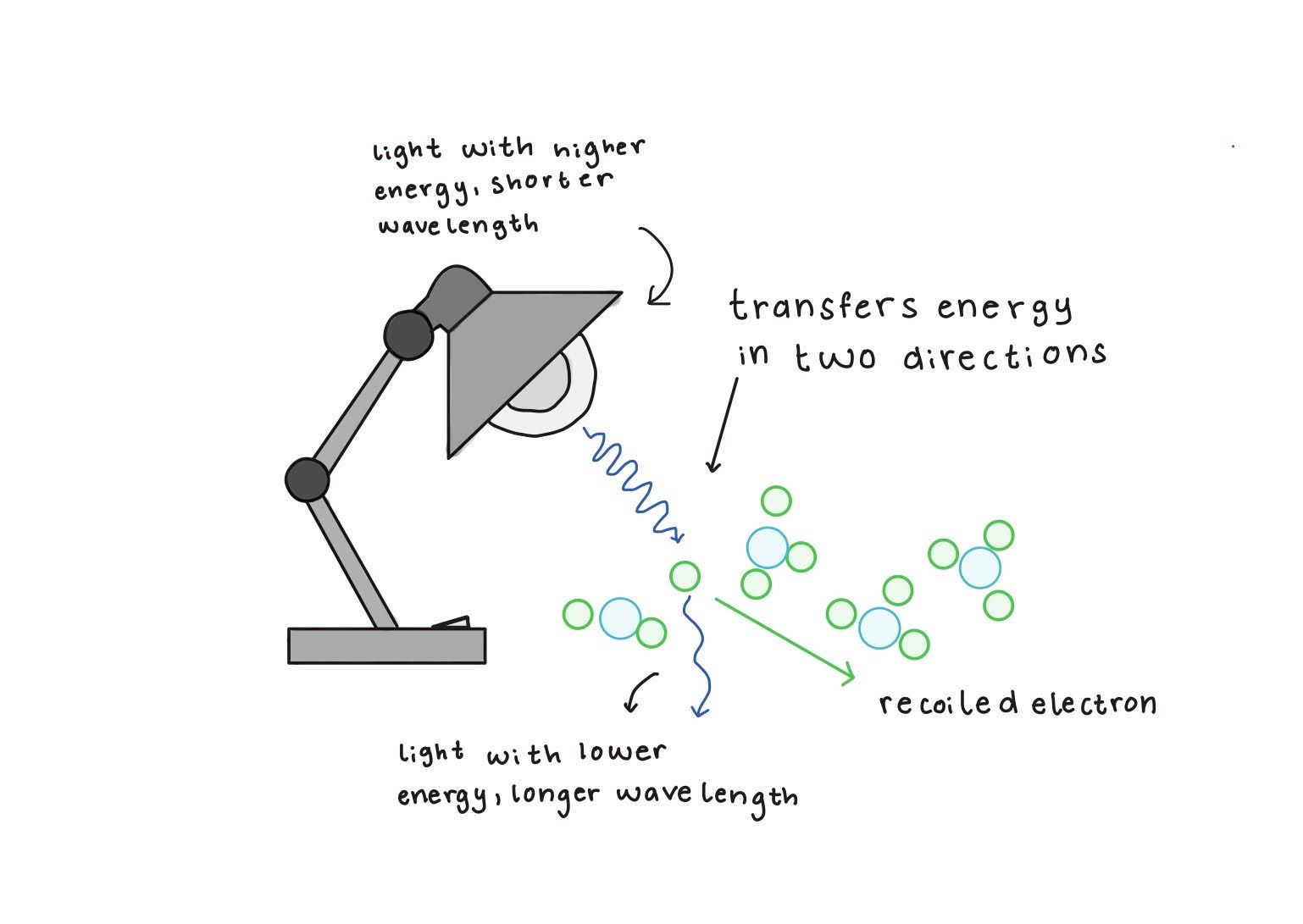
Another major blow to the wave theory of light came from the photoelectric effect, first observed by Heinrich Hertz in 1887, when ultraviolet light caused electric charge to be emitted from a metal surface (see Figure 1.2). By 1900, Philipp Lenard conducted detailed experiments to show that the energy of ejected electrons depended on light frequency, not intensity, an observation that classical wave theory struggled to explain. In 1905, Albert Einstein addressed this by proposing that light is made of discrete energy packets, now called photons, each carrying an energy proportional to its frequency according to Planck’s quantization rule. This idea successfully explained the photoelectric effect and was later confirmed by Robert Millikan’s experiments in 1915, which verified that the energy of the ejected electrons depends on light frequency and that intensity affects only the number of ejected electrons. Einstein’s explanation earned him the 1921 Nobel Prize in Physics. The existence of the photon was further reinforced in 1923 by American physicist Arthur Compton, who showed that X-rays scatter off electrons and emerge with smaller frequencies (see Figure 1.2).
Wave-Particle Duality (1924 – present)
The photoelectric effect showed that light interacts with matter through discrete, fundamental processes, behaving as if it were made up of particles known as photons. On the other hand, Young’s double-slit experiment provided convincing evidence that light also exhibits wave-like behavior through interference. These seemingly contradictory findings reveal the dual nature of light, a concept known as wave-particle duality. Today, this principle is central to quantum physics, which describes light as a quantum electromagnetic field. This field has discrete energy excitations called photons that can produce particle-like effects, while also exhibiting wave-like behavior depending on how light interacts with its environment.
References
1. Hewitt, P. G. (2015). Conceptual physics (12th ed.). Pearson Education, Inc.
2. Cohen-Tannoudji, C., Diu, B., & Laloë, F. (1977). Quantum mechanics: Volume I (S. R. Hemley, N. Ostrowsky, & D. Ostrowsky, Trans.). Wiley-Interscience/Hermann.
3. Rooney, A. (2011). The story of physics: From natural philosophy to the enigma of dark matter. Arcturus Publishing Limited.
4. William Harris & Craig Freudenrich, Ph.D. “How Light Works” 1 January 1970. HowStuffWorks.com. <https://science.howstuffworks.com/light.htm> 7 July 2025
Written by Richard Sottie, a graduate student in the Physics & Astronomy program at Ohio University, specializing in computational physics with a focus on plasmonics.
Illustration created by Serena Krejci-Papa, a first-year master’s student at the University of Barcelona studying theoretical and computational chemistry with the Erasmus Mundus program. She writes about complex science topics in a way that makes people laugh. You can find more about her at Sciencewithserena.com.
For general questions about IYQ, please contact info@quantum2025.org. For press inquiries, contact iyq2025@hkamarcom.com.