How Does Quantum Help Us Understand Chemistry?

We talked before about how the word “quantum” often appears alongside the word
“physics,” but that quantum science is also important to fields like chemistry. Is quantum science used in chemistry?

That’s a great question! A lot of people learn about chemistry in school without understanding that quantum science lies at the heart of how and why atoms stick together to form molecules and materials. For example, consider the simplest and smallest atom, hydrogen. If you have a bottle filled with just hydrogen gas, the hydrogen atoms in the bottle aren’t bouncing around by themselves; they like to pair up with each other to make hydrogen molecules.

Yes, that’s the difference between a hydrogen atom and a hydrogen molecule; the molecules are paired-up atoms. The same thing is true of oxygen, too, isn’t it?

That’s right—oxygen molecules in the air around us that we breathe are bound together in pairs. In addition to hydrogen atoms sticking together and oxygen atoms sticking together, you can also get combinations of hydrogen and oxygen atoms.

I know one: water! H₂O—two hydrogen atoms and one oxygen atom form chemical bonds with each other to make one water molecule.

Exactly. There’s one other compound you can make out of hydrogen and oxygen, hydrogen peroxide, which is a combination of two hydrogen atoms and two oxygen atoms, H₂O₂, and is used to bleach things, like paper, to make them white. This compound isn’t as stable as water; in fact, over time, it tends to fall apart, and any other combination you make of hydrogen and oxygen will quickly fall apart.

Why is this? Why does one oxygen atom like to stick to exactly two hydrogen atoms and not just one, three, or seven? Why do oxygen atoms like to pair up with each other rather than be apart or in groups of three or some other number?

These are excellent questions that have puzzled chemists for many years. Elements like hydrogen and oxygen were first isolated and named in the late 1700s. The 1800s saw the development of the idea that all compounds were whole-number combinations of chemical atoms; however, a mystery remained as to why certain combinations of atoms were allowed and others seemed forbidden.

So, did it just seem random which combinations worked and which ones didn’t?

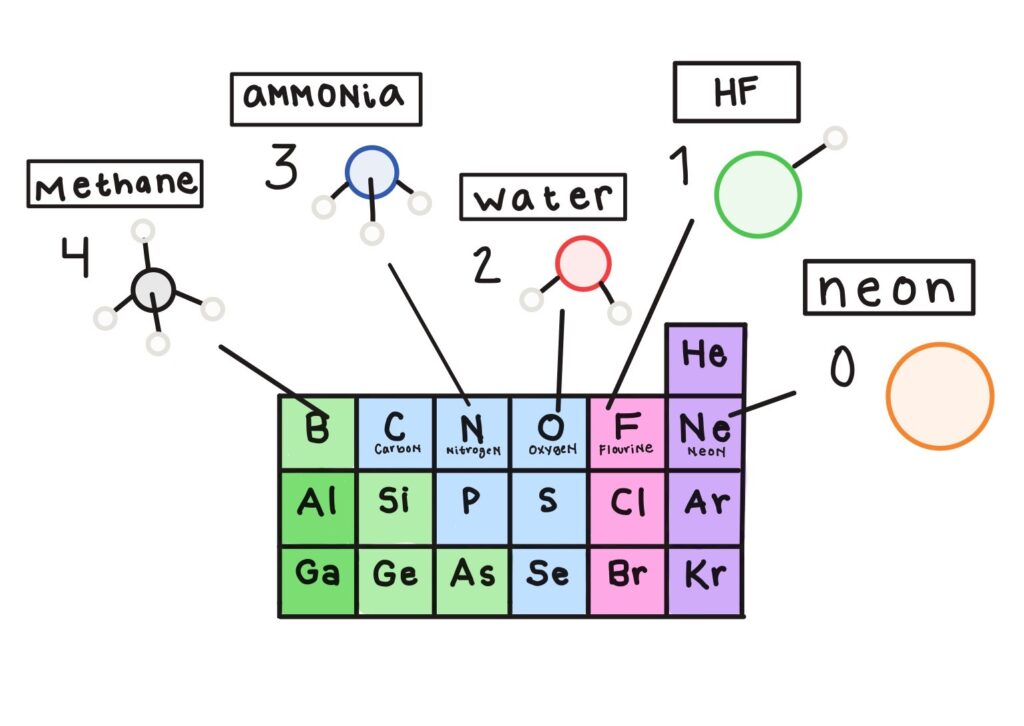
Not at all. From doing experiments and combining elements, chemists noticed certain patterns about how atoms combined. For example, when the elements were organized into the periodic table according to similar chemical behavior, the fact that there are eight elements in the second row matched up with the observation that elements along this row liked to make a certain number of bonds depending on their position in the row. For example, carbon, which is the 4th element in the row, likes to make four bonds; nitrogen, which is the 5th element, likes to make three bonds; oxygen, which is the 6th element, likes to make two bonds; fluorine, which is the 7th element, likes to make one bond; and neon, which is the last element in the row, doesn’t like bonding to anything.

So oxygen, in the 6th position, likes to make bonds with two hydrogens to make water. I see the pattern you’re talking about: 6 + 2 = 8. Why eight?

This is exactly the question chemists were pondering at the start of the 20th century. There was clearly some reason behind this rule of eight, or “octet rule,” but no one understood where this eight came from. One interesting idea was that a cube had eight corners, so maybe there was something cubical about atoms that made them want to have one electron at each corner of the cube, which they could achieve by sharing electrons. But there was no evidence that there was anything cubical about the arrangement of electrons in atoms, so that model wasn’t the solution to the puzzle about the rule of eight.

So what did solve the puzzle, then?

Quantum mechanics! Almost as soon as quantum mechanics was developed, starting one hundred years ago, scientists saw how applying it to the problem of how atoms were structured—a positively charged nucleus attracting electrons to it—led directly to the patterns of the periodic table. It explained not only the rule of eight, but all sorts of other rules for how and why atoms chemically bond together. Soon, chemists not only had a quantum understanding of why oxygen likes to bond to two hydrogens to form water, but also used quantum science to find rules governing chemical combinations, compounds, and bonds that they hadn’t previously understood.

But how did quantum mechanics explain this rule of eight?

Remember that the “quantum” in quantum mechanics means something you can count. A hallmark of quantum science is showing how there are sometimes countable aspects to things that don’t seem on the surface like there’s anything there to count. In the case of atoms and bonds, the attractions and repulsions of electrons and nuclei seemed like a problem where there wouldn’t be anything countable about the possible arrangements of the electrons and the bonds they form. It was only with a quantum understanding of the wave-like nature of electrons that the hidden counting of these arrangements was revealed.

So, thinking about it, every single bond between every single atom, holding together all the materials and objects, is governed and described by quantum mechanics.

Exactly, not only all the things around us, but us as well! We wouldn’t understand how the atoms in our bodies stick together without quantum mechanics. Quantum mechanics solved some of the mysteries from a century ago about how simple compounds work, but even today, researchers are actively using quantum mechanics to reveal how more complicated materials and molecules work – including many of the ones that make up you and me.
Written by Paul Cadden-Zimansky, Associate Professor of Physics at Bard College and a Global Coordinator of IYQ.
IYQ mascot, Quinnie, was created by Jorge Cham, aka PHD Comics, in collaboration with Physics Magazine.
All rights reserved.
Illustrations: Serena Krejci-Papa
Featured image: Electronics factory worker, Cikarang, Indonesia © ILO/Asrian Mirza
For general questions about IYQ, please contact info@quantum2025.org. For press inquiries, contact iyq2025@hkamarcom.com.